The Science of Homeopathy
Abstract: Homeopathy is a centuries-old system of medicine which has been a source of controversy and debate in scientific medical circles since its origin. Studies demonstrate consistently high patient satisfaction rates, but outcomes from randomized controlled trials of homeopathy remain inconsistent. This discrepancy may reflect a failure of the method applied to judge homeopathy, the Randomized Controlled Trial. The benefits of homeopathy appear to cover a broad range of conditions. The safety profile of homeopathic treatment is excellent. Homeopathy is worthy of further scientific investigation and implementation.
Keywords: Homeopathy, Observational Studies, Randomized Controlled Trials, Meta-analyses, Safety Profile.
A- The Conventional Crisis
Conventional allopathic medicine is in a state of crisis. Dissatisfaction is growing among both physicians and patients. “A growing chorus of discontent suggests that the once-revered doctor-patient relationship is on the rocks….many patients don’t trust doctors.”
Physicians are also at odds. The president of the Medical Society of the State of New York remarked: “As we struggle to adjust to an increasingly hostile practice environment, it is the medical profession itself that is in need of a doctor.”
At least 25% of patients feel that their doctors aren’t looking out for their best interests. They express concerns that their physicians expose them to “unnecessary” risks, act like they “know everything”, fail in communication with them, are increasingly isolated, have differing treatment goals: physicians are interested in making diagnoses, while patients are interested in being tended to, listened to, cared for, and being well.
This breakdown in the physician-patient relationship is a symptom of a much larger situation: The culture surrounding medicine has changed, but the practice of medicine has not kept up. The factors include managed care, third party payers, declining reimbursements, increasing malpractice liability insurance costs, rising overhead, and increasing physician debt.
A growing population of disgruntled physicians worsens the conventional health care system’s struggle to provide reasonably affordable care to an increasingly unhealthy population. Physicians grapple with the grim realities of a failing practice environment and are estranged from their patients who try to second-guess their thinking and propose their own diagnoses. The pressures placed on practicing physicians have forced many to spend less time with their patients and others to seek alternative treatment strategies that might provide safer, more effective and satisfying care.
A recent survey predicts that “nearly half of the nation’s primary care physicians plan to stop practicing or reduce patient load” in the three years. Physicians cite frustration over non-clinical paperwork, difficulty receiving reimbursement and burdensome government regulations. State medical societies and government agencies warn of inevitable primary care physician shortages that are worsening. Among primary care physicians, a 14% shortfall is expected by 2030.
Administrative and medical-legal concerns have pressured physicians to become more defensive. Decreasing reimbursement rates and demanding managed care requirements have forced many physicians to either see more patients in less time or to leave the system entirely. The physician-patient relationship is taxed, weakening their already tenuous channels of communication and leading to greater tensions between patient and physician.
Despite significant advances in health care, discontent with conventional medicine is growing. Technological solutions may temporarily stem the tide, but they only delay the inevitable deterioration of this relationship. In the U.S., more money is currently spent “out of pocket” for complementary and alternative medicine than for conventional medicine. In a country that spends more per capita on medical costs than any other nation on earth, this is a serious sign of trouble.
“Physician extenders” or “mid-level practitioners” increasingly fill the gap in a system that is squeezing physicians out of the equation. As a result of the widening breach in the physician-patient relationship the practice of conventional allopathic medicine is becoming untenable. Alternative solutions and approaches need to be explored.
In sharp contrast to this scenario, a recent study conducted by the Swiss government over seven years demonstrated that homeopathic medical care was superior to conventional methods in several respects. The study showed that patients’ quality of life and satisfaction with the doctor-patient relationship were both significantly higher when homeopathic treatments were utilized. At the same time, health related costs were reduced by 50% with the use of homeopathy.
In the United Kingdom a six year follow-up study of 6,544 patients receiving homeopathic treatment in the National Health Service (NHS) arrived at similar results. Outcome data showed that homeopathic intervention was beneficial in a substantial proportion of patients with a wide range of chronic diseases. The authors concluded that: “Additional observational research, including studies using different designs, is necessary for further research development in homeopathy.”
A study at the Royal London Homeopathic Hospital found a clinical improvement rate of 81% with a 90% patient satisfaction rate in patients treated homeopathically.
Homeopathy is an individualized, psychosomatic system of medicine based on more than two centuries of clinical observation, and carefully conducted trials. It relies on an extensive data-base of clinical treatment; evidence built on successfully treated patients, clinical and toxicological research. It differs from conventional medicine in several key areas:
- Homeopathy postulates a psychosomatic body-mind connection not described by the allopathic medical model.
- Homeopathic treatment provokes an innate healing response in the body that is disputed by conventional medical theories.
- Homeopathy adjusts its approach and individualizes treatments to individual patients.
- Homeopathy utilizes natural medicines, many of which have been in continual use for over two hundred years.
- Homeopathy recognizes that extremely minute dilutions of a substance can have profound healing effects.
- Homeopathy is based upon direct clinical research that incorporates both objective and subjective viewpoints.
Laboratory research in homeopathy has lagged far behind conventional medicine largely because most practitioners believed it was unnecessary in the face of direct clinical evidence of effectiveness in cured cases. Observational studies of homeopathic practice document consistently strong therapeutic effects and sustained satisfaction in patients.
Homeopathy offers a unique solution to the growing crisis facing conventional allopathic medicine. Opposition to homeopathy has always asserted that there is no proof that it is effective. However, objective review of data from studies reveals abundant evidence to support homeopathy, showing that the practice warrants further study. The following sections discuss why some of the impediments to this process have prevented fair research into homeopathy and why it should be considered in more depth.
B- Evidence Based Medicine
“Legitimate standards of medical practice are rooted in competent and reliable scientific evidence and experience. However, these standards are subject to continual change and improvement as advances are made in scientific investigation and analysis.”
Evidence Based Medicine (EBM) means: “integrating individual clinical experience with the best available external clinical evidence from systematic research.”
A group of authors have criticized homeopathy and other forms of CAM, labeling them “quackery”, in part because they appear to lack an evidence base. These writers fail to recognize that this apparent lack of an evidence base is even more of a problem with respect to conventional allopathic therapies.
A group of information specialists and statisticians at the British journal BMJ Clinical Evidence, who reviewed over 2,500 conventional allopathic treatments in 24 categories (see Figure 1) of illness, found that:
- Only 13% of conventional treatments were “beneficial” when the clinical evidence supporting their use was investigated,
- Only 23% were “likely to be beneficial”, but evidence was inconclusive,
- 8% were “a trade off between benefits and harms”,
- 6% were clearly “unlikely to be beneficial”,
- 4% were “likely to be ineffective or harmful”,
- 46% were rated as being of “unknown effectiveness”. The clinical data supporting their use was simply nonexistent.
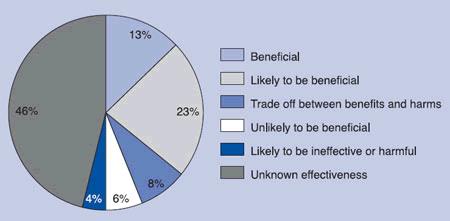
This figure shows that only 13% of all conventional allopathic medical treatments employed today are supported by scientific evidence. The vast majority (87%) of conventional therapies lack any scientific evidence base.
The BMJ authors concluded that most conventional medical treatments do not rest on principles of evidence based medicine (EBM), but on the “individual preferences of clinicians”, unsupported by science. This finding shows that the label of “scientific medicine” is improperly applied when it is used in conjunction with conventional allopathic practices. Only a small percentage of treatments in the allopathic armamentarium are backed by evidence of consistent beneficial results.
In 2001 researchers looking at data used to support conventional medical practice uncovered an additional problem. Re-analyzing 160 conventional studies initially performed by the prestigious Cochrane Institute these authors concluded that the scientific evidence cited in support of many practices was far less robust than the initial reviewers concluded. They learned that the Cochrane interpretations tended to be “highly subjective” in their analyses. In other words, many of the studies that were cited as scientific justification for particular conventional practices did not actually support, or only weakly supported, the treatments in question. They learned that the original reviewers had adopted unjustifiably favorable positions on a large number of therapeutic interventions. They found that in these instances the conclusions drawn from the Cochrane reviews were fundamentally flawed and prejudicially in favor of these therapies.
This data shows that the claim to an evidentiary basis for allopathic medicine is biased and seriously flawed. Numerous reports show that scientific data interpretation is frequently flawed. Some research results have been fabricated by investigators. In other instances unfavorable information was simply withheld from publication. Pharmaceutical companies routinely “bury” negative results, disguise negative studies inside more positive ones and continually “blur the lines between science and marketing.” Studies performed by researchers financially linked to conventional pharmaceutical companies have been shown to have consistently more positive results that those published by independently funded researchers.
An expert at the Johns Hopkins School of Public Health recently analyzed the research and publication strategies of the Pfizer pharmaceutical company finding:
“Pfizer’s tactics included delaying the publication of studies that had found no evidence the drug worked for some other disorders, “spinning” negative data to place it in a more positive light, and bundling negative findings with positive studies to neutralize the results…”20
More than half of the clinical trials conducted for drugs approved by the FDA are never published. Only 43% of drugs approved by the FDA between 1998 and 2000 had published trial results. The FDA has been cited for its laxity in ensuring that drug trials are conducted properly. Studies suggest that 33% of physicians overseeing drug trials have a conflict of interest and receive payment from pharmaceutical companies. Failure to report these conflicts results in covert bias. Marketing strategies using the claim of “scientific” in a specious way further muddies the waters. These conflicts of interest distort scientific objectivism for the economic benefit of the pharmaceutical industry and places public health and safety at risk.
Other evidence of tangled science and marketing strategies include:
- The drug manufacturer Wyeth recently admitted paying ghostwriters to produce medical journal articles favorable to its products.
- The manufacturer Merck recently “downplayed” dangers of its drugs, concealed the true authorship of articles and hired ghostwriters without revealing their financial ties to the industry. Reviewers agreed that these events required the collusion of “researchers, authors, journal editors, peer reviewers and the FDA.” They concluded that: “Public trust in clinical research is in great jeopardy.”
- GlaxoSmithKline pharmaceuticals recently revealed they had paid over $1.3 million to an influential psychiatrist who hosted a National Public Radio health program. The support was not divulged publicly and the psychiatrist neglected to report the income to his university affiliate.
- A recent congressional investigation revealed that several drug manufacturers, including Johnson & Johnson, paid large sums of money to world-renowned physicians for public advocacy of their products on a large scale.
These actions reveal a type of covert lobbying effort that clearly violates the public trust. This raises the question of the safety over the entire conventional medical armamentarium and reveals that the conflict of interest between manufacturers and physicians is extremely serious. The entire foundation of conventional allopathic pharmaceutical based medicine needs to be seriously reexamined.
Classical Homeopathy, on the other hand, is not subject to the same forces of industry-based profit motives. Homeopathic treatment must be, by definition, individually prescribed and matched to specific individuals. Homeopathic medications are natural products that are prescribed as simple and inexpensive extractions and dilutions. These products are generally given as single medicines, and repeated only infrequently. Since homeopathic medicines do not involve alterations of natural substances, they are not patentable. This simple factor reduces the profit motive dramatically and makes the field a likely target for disparagement by the profit based pharmaceutical industry (which needs to claim exclusive effectiveness in order to convince the public of the value of buying its products).
The evidence base of homeopathy is solid. It rests in clinical practice, not marketing techniques or studies in large multi-center trials. Homeopathic proof is built upon a combination of direct evidence through reports of cured cases, clinical provings, and toxicity studies.
The clinical proving is the process whereby each homeopathic medicine is administered to healthy volunteers and their symptoms are recorded.
Data from toxicity reports and poisonings are also included since they add to the drug picture’s full spectrum of activity.
Homeopathy is built upon a centuries-old collection of evidence that integrates the subjective clinical experience of the patient along with the objective clinical observations of the physician.
Its practices involves administering individually selected homeopathic medicines that most closely match the totality of symptoms of the sick patient in a highly dilute (“potentized”) form. Homeopathic drug information (and homeopathic prescribing) is based on the empiric science of clinical observation. This data has been repeatedly confirmed and substantiated over more than two centuries. It demonstrates internal consistency unbiased by internal individual or external market forces.
Allopathic medical prescribing is supposed to be based upon objective clinical observation, but it has been heavily corrupted by the pharmaceutical industry, and an “academic industrial complex” representing world-wide financial interests in the trillions of dollars. The ethics, science and objectivity of the industries involved is riddled with conflicts of interest and has a long history of covert and overt abuse of the public trust.
Homeopathic and allopathic routes diverged over the last two centuries. This period has been marked by fierce disagreement, rivalry and outright hostility between the groups. Over the years the face of allopathic medicine has changed dramatically as it assumed a central role in our nation’s health care. On the other hand, homeopathy has barely changed at all in the past two centuries. Its practice today is bolstered by many scientific advances, but at its root, it maintains the same philosophy, medicines and method of practice. This failure of homeopathy to change may, in fact, represent a form of proof that the system is extremely successful, safe and effective. If this is the case, we must ask how it is that these results have not been accepted by the public? The answers lie in the obstacles to homeopathic research when it attempts to conform to the standards of allopathic research.
C- Ideological problems facing research in Homeopathy
Homeopathic research is not accepted by mainstream medical institutions today mainly due to ideological conflicts, misunderstanding and prejudice, not lack of clinical effectiveness.
Because homeopathy lacks any clear explanation for how it might work it has been repeatedly derided and ridiculed by proponents of the mechanistic medical model. The problems endured by homeopaths trying to prove the effectiveness of their practice to allopathic colleagues stem mostly from prejudicial and ideological bias, not lack of clinical proof. Allopaths simply do not believe that homeopathy could work:
“The problem with homeopathy is that the ‘infinite dilutions’ of agents used cannot possibly produce any effect. A randomized trial of ‘solvent only’ versus ‘infinite dilutions’ is a game of chance between two placebos.”
Allopathic “scientists” frequently refuse to even look at, let alone accept, what the science of their own studies on homeopathy demonstrates. They refuse to look at the data because they have already pre-judged and made up their minds that homeopathy is “simply not possible”. This attitude does not represent scientific inquiry, but dogmatic rigidity.
Despite many reasonably good studies performed on homeopathy, and a tremendous amount of observational data from patients, the allopathic community continues to stubbornly insist that homeopathy cannot possibly work. Hard data showing homeopathy’s effectiveness is met with mockery, dismissal and outright refusal to consider the implications.
One investigator remarked:
“Either the studies show what they seem to show– that homeopathy is working– or they demonstrate the Random Controlled Trials capacity for predictable, reproducible, significant false positives — a conclusion that may be even more challenging in its implications for today’s medicine than the conclusion that homeopathy works.”
Conventional allopathic medical researchers are simply too prejudiced against homeopathic practice and research to accept its validity. Allopaths have continually refused to seriously consider the scientific data. Their reasoning that further research would be futile is justified by the argument that homeopathy is simply implausible:
“…the scientist must question whether the diversion of significant resources to support these trials [of homeopathy] can be justified when a rational basis for choice of homeopathy, or any particular modality of it, is lacking.”
As a result of this prejudice, financial support for homeopathic research and academic appointments has suffered. Medical history in the United States has been unequivocal on this subject: The American Medical Association was founded, in part, to fight an ideological and financial “turf war” against homeopathy. Until medical scientists are able to suspend ideological prejudice and look objectively at the results of all medical trials, there will be continued corruption in medical thinking and no greater acceptance of effective scientific disciplines like homeopathy in the future.
D- Practical problems facing research in Homeopathy
Apart from the huge ideological gap separating homeopathic from allopathic medical thinking there are still many practical obstacles facing the study of homeopathy:
- Lack of Funding. A double-bind exists here since funding is usually limited to therapies that already have a proven track record of effectiveness. Obtaining funding for homeopathy is more difficult due to the bias against homeopathy. Gaining the necessary financing to prove itself is twice as difficult when access to insurance coverage and research funds are limited. In the UK only 0.08% of the NHS research budget is devoted to CAM.9 In the U.S. 0.4% of the total operational budget of the NIH is devoted to the National Center for Complementary and Alternative Medicine (NCCAM). (Only two departments of the NIH receive lower funding than NCCAM, one of them being “Building and Grounds”.) The NCCAM budget is further divided between a diversity of CAM modalities, including homeopathy.
- Lack of Profit. Homeopathic research suffers from a lack of profitability precisely because these medicines are inexpensive to produce and test. The homeopathic pharmacopoeia has changed very little since its inception, unlike the allopathic armamentarium, which requires constant annual revision. The single-ingredient homeopathic medicines, derived primarily from natural sources, can be produced, tested, developed and manufactured relatively inexpensively. Profit margins are extremely small in homeopathy. As a result, profit does not drive homeopathic research nor attract large investments, as it does in the allopathic pharmaceutical industry. The main incentive to support homeopathy arises from its health benefit not its investment return. Consequently, philanthropic support has dominated homeopathic funding.
- Lack of Research Skills. Most homeopathic schools and training programs lack consistent standards or academic rigor, and most fail to offer electives or training in research. In the U.S. there is no centrally designated authority governing the academic program in these schools. The Council on Homeopathic Education (CHE) has attempted to unify and improve these standards, but there is still a long way to go.
- Lack of an Academic Infra-structure. Homeopathic training programs lack connection with university training programs or medical schools. This limits funding, support and visibility in the academic community. The American Medical College of Homeopathy (AMCH) in Phoenix, AZ is an example of an attempt to correct this situation but it remains under-funded and unaccredited. Only six universities in the United Kingdom (and none in the U.S.) currently offer Bachelor of Science degrees in homeopathy. Homeopathic medical schools imparting the M.D. degree still exist only in India. Only naturopathic schools formally offer accredited training in homeopathy in the U.S. and only at introductory levels.
- Lack of Patients. According to recent studies between 1 – 3% of the U.S. population has utilized homeopathy.8 This number is growing steadily, but the public is still largely unaware of the benefits of homeopathic treatment. This is in stark contrast to the situation in the late 19th century, when Homeopathy enjoyed wide public support, a broad political backing and an academic infrastructure with homeopathic medical schools, hospitals and clinics across the U.S.
- Lack of Practitioners. Medical practitioners typically provide direct patient care, but also form the pool of researchers who generate clinical data. Research performed by homeopathic clinicians who have chosen to investigate some aspect of homeopathic treatment in their private practices is extremely limited. Existing practitioners who elect to perform such research find themselves spread extremely thin since the number of homeopathic physicians in the U.S. is very low. The American Institute of Homeopathy (AIH), the flagship professional homeopathic organization, currently boasts less than 200 physician members nationwide.
E- Methodological Problems in Homeopathic Research
Besides the ideological and practical problems that prevent homeopathy from gaining greater acceptance and recognition, structural problems in the current philosophy of medical science add to the difficulty of objective validation of homeopathy.
Homeopathy was the first medical discipline to utilize statistics in the analysis of therapeutic efficacy. Samuel Christian Hahnemann, MD, the founder of homeopathy, was one of the first physicians in modern times to look critically at medical therapeutics and to demand evidence with respect to effectiveness and therapeutic efficacy.
The “gold standard” of homeopathic medicine is direct clinical experience, initially through the clinical proving, and subsequently by attending to clinical outcomes; the resolution of disease states following individualized homeopathic treatment.
Allopathic medicine considers the Randomized Controlled Trial (RCT) to be the “gold standard” of medical research. However, since this is based on massing large amounts of data on the basis of diagnostic categories of diseases, it has limited applicability when applied to homeopathy, which is based on individualized treatment and does not group patients into disease-diagnosis categories.
Homeopathy is not focused on whether a particular medication statistically impacts a disease category. It is interested in finding the right remedy for the individual person. However, each person is unique, as is their affliction, obviating the value of research based on categories of disease. Holding the RCT as the “gold standard” in such a circumstance automatically discredits homeopathy from the outset and is the root problem blocking the objective validation of homeopathy in medical research.
- Homeopathy cannot be studied through randomization without loosing its unique advantage. It is difficult to apply the RCT to homeopathy because homeopathic treatment is (by definition) individualized to the person, not the diagnosis or the condition. Finding the cure for individual patients with their individual illness is based on the explicit position that they are unique and do not fall into groups. Patients in homeopathic treatment cannot be compared or randomized into treatment/non treatment settings the same way as patients in groups representing disease categories can be. Homeopathy is not applied on the basis of a diagnostic category in practice, so studies of homeopathic treatment based upon analysis of results with disease categories is meaningless. Homeopathy requires a separate in-depth interview and psychosomatic analysis of each individual case to determine the correct individual prescription. The basic premise of the RCT approach is in conflict with the basic premise of homeopathy.
According to the Liga Medicorum Homeopathica Internationalis: “any work that aims to demonstrate the evidence of Homeopathy based on clinical trials designed to prove efficacy of a particular homeopathic medicine exclusively over a specific disease will fail… because it ignores the intrinsic homeopathic principles”.
- The RCT is not sensitive to individuals. These studies typically test a single medicine on a large number of patients with a single diagnosis. This approach ignores the way that homeopathy is practiced, which is by individualizing medicine choice to the unique and total clinical pattern of each patient. The RCT is an example of linear reductionism which eliminates the differences between patients, but homeopathic treatment is actually based upon accentuation of those very differences! The RCT therefore cannot be an adequate test of the effectiveness of the homeopathic approach as it deliberately eliminates the complexity and individualization inherent to the homeopathic prescription.
- The RCT tests simplified objective events and filters out detailed information about how individuals fare from treatment. Homeopathic treatment makes a difference in precisely those criteria that are not measured in the RCT: quality of life, long term measures of improvement, energy and vitality. The endpoints of homeopathic and allopathic treatment are different. Observational studies of homeopathic clinical outcomes are what is needed to evaluate the efficacy of homeopathy, not RCT’s.
- The RCT is not based on real life experience, real-life conditions or practical considerations of patient’s daily lives. Conditions investigated in clinical trials are rarely representative of those treated in actual practices. Researchers typically select conditions that are relatively easy to investigate and recruit large numbers of patients. Researchers select simple cases that have one or two easily measurable short-term outcomes. This is frequently not representative of the populations treated in clinical practice where cases are always more complex and varied.
Judging homeopathy by the RCT approach is an inappropriate and inadequate measure of homeopathy’s true potential. Statistical trends can be measured by the RCT, but not a system of medicine based upon the complexities of the individual.
F- Evidence from Randomized Controlled Trials
Despite these ideological, practical, and methodological barriers to research in homeopathy, many investigations, including RCTs, have still demonstrated that homeopathy is effective.
Randomized Controlled Trials are by their nature limited in scope focusing on short-term outcomes in an externally imposed time frame. These studies fail to evaluate the long-term benefits of homeopathy.
Recent studies, including individual RCT’s as well as Meta-Analyses, have tested homeopathy across a broad range of conditions. Unfortunately, even though the majority of these studies continue to demonstrate some form of design flaw or methodological weakness, these shortcomings are starting to be addressed as the accumulation of positive findings spurs more thorough research. Study designs are improving in the most recently published trials.
The following is a brief summary of several recent studies:
• Asthma ,
Twenty eight patients with allergic asthma were randomly assigned to receive oral homeopathic immunotherapy (Isopathy) to their principal allergen or to placebo. Patients were assessed after 4 weeks of treatment. Homeopathically treated patients showed significant improvements on a visual analogue score, in respiratory function and bronchial reactivity, benefits that persisted at eight weeks.
• Eczema
One hundred and eighteen children were randomized to receive either homeopathic (n=54) or conventional (n=64) treatment of eczema for 12 months. Disease related quality of life improved similarly in both groups.
• Childhood Diarrhea ,
Eighty-one children with diarrhea were randomized to receive either an individually chosen homeopathic medicine or placebo. The homeopathically treated group had an earlier statistically significant reduction in duration of diarrhea compared with controls.
• Acute Otitis Media , ,
Two hundred and thirty children with AOM received an individualized homeopathic medicine in the pediatric office. Pain control was achieved in 39% of the patients after 6 hours, and an additional 33% after 12 hours. This resolution rate was 2.4 times faster than in placebo controls. There were no complications observed in the study group, and compared to conventional treatment the approach was 14% cheaper.
• Hay Fever and Allergic Rhinitis ,
Fifty-one patients with perennial allergic rhinitis were treated with either a homeopathic preparation of the principal allergen (Isopathy) or placebo. After 4 weeks the homeopathic group had 21% improvement in symptoms compared with 2% improvement in the placebo group. The homeopathic group reported initial aggravation in symptoms more often than the control group. The authors noted: “compared with placebo, homeopathy provoked a clear, significant, and relevant improvement in nasal inspiratory peak flow, similar to that found with topical steroids.”
• Influenza ,
Four hundred seventy eight patients with the flu were given either Oscillococcinum or placebo. The proportion of homeopathic patients who recovered within 48 hours was significantly greater than those receiving placebo.
• Muscle Soreness
Eighty-two marathon runners were given either Arnica or placebo daily beginning the day before running. Muscle soreness was significantly lower in the Arnica group when compared with the placebo group immediately after the marathon.
• Pain ,
Forty-three patients suffering from chronic low back pain were randomized to receive either homeopathy or standardized physiotherapy. The homeopathically treated group demonstrated a significant decrease of the pain after treatment compared with the PT treated group. The authors concluded that: “nothing can be said against attempting treatment of chronic low back pain by means of homeopathy.”
• Radiotherapy Side Effects ,
Sixty-six patients received either homeopathic medicine or placebo during radiation therapy following breast cancer surgery. The homeopathic group showed statistically significant improvement in breast skin color, warmth, swelling and pigmentation over 10 weeks compared with the control group.
• Rheumatoid Arthritis
Twenty-three patients with rheumatoid arthritis were treated with either a complex homeopathic preparation or placebo. After 12 weeks the homeopathic treated group showed a significant improvement in pain, movement, inflammatory signs, morning stiffness, and fatigue compared with the placebo group.
• Tissue Trauma
Sixty patients with mild traumatic brain injury received either an individually assigned homeopathic medicine or placebo. Homeopathically treated patients demonstrated a significant improvement compared with placebo treated patients. The authors found that homeopathic treatment was “the only significant or near-significant predictor of improvement” and “homeopathy may have a role in treating persistent Mild Traumatic Brain Injury (MTBI).”
• Respiratory Infections , ,
Four hundred and fifty six patients with upper respiratory tract infections (URI), lower respiratory tract infections and ear complaints were treated by conventionally trained physicians. Of these, 175 were treated conventionally: 71% received antibiotics and 33% were given cough/cold preparations. The remaining 281 were treated homeopathically, receiving a variety of remedies. Cure or major improvement was achieved in 82.6% of the homeopathically treated patients after 14 days versus 68% of those conventionally treated. Adverse events were 7.8% in the homeopathy group versus 22.3% in the conventional group. Patient satisfaction was 79% in the homeopathy group versus 65% in the conventional group. The authors concluded that: “Homeopathy appeared to be at least as effective as conventional medical care in the treatment of patients with the three conditions studied.”
Evidence from these studies is sufficient to suggest that homeopathy offers some clinical validity, making it worthy of significantly more clinical, academic and laboratory investigation.
G- Evidence from Meta-analyses
Meta-analyses systematically review and compare pooled results from numerous studies for a given condition or therapy. The following is a summary of the most recent meta-analyses of homeopathic treatment.
• British Medical Journal 1991.
These researchers looked at 107 trials of homeopathy. Of these only 105 could be analyzed. Eighty one had positive outcomes and the authors were able to determine that homeopathy was superior to placebo 68% of the time. The authors concluded: “The evidence presented in this review would probably be sufficient for establishing homeopathy as a regular treatment for certain indications…Based on this evidence we would be ready to accept that homeopathy can be efficacious, if only the mechanism of action were more plausible” (emphasis added).
• European Commission Report 1996.
These researchers looked at a total of 21 studies (out of 377 available) and concluded that: “there is an activity of homeopathic remedies higher than the placebo… these conclusions confirm entirely the result of prior audits. Homeopathy is therefore worthy of research.”
• Lancet 1997.
These researchers looked at a total of 89 RCTs. These authors found that 73% of trials demonstrated that homeopathy was more effective than placebo. The results showed an odds ratio in favor of homeopathy of 2.45 with a 95% confidence interval. (The odds of improving from homeopathic treatment were 2.5 times greater than with placebo.) This difference was statistically significant. The authors concluded that the: “results are not compatible with the hypothesis that the clinical effects of homeopathy are completely due to placebo.”
• Journal of Complementary and Alternative Medicine 1998.
A total of 32 trials involving 1778 patients were reviewed. In 19 of the placebo-controlled trials individualized homeopathy was significantly more effective than placebo (pooled rate ratio 1.62, 95% confidence interval). The authors concluded that: “The results of the available randomized trials suggest that individualized homeopathy has an effect over placebo. The evidence, however, is not convincing because of methodological shortcomings and inconsistencies.”
• European Journal of Clinical Pharmacology 2000.
These authors analyzed 16 randomized controlled trials of homeopathy and concluded that: “It is likely that among the tested homeopathic approaches some had an added effect over nothing or placebo”, and that: “at least one [of the tested homeopathic treatments] shows an added effect relative to placebo.” The group recommended that homeopathy be studied further using the same methods used to study conventional medicine.
• Annals of Internal Medicine 2003.
The authors reviewed a number of meta-analyses and other systematic reviews and concluded that “there is positive evidence for overall effect, but the limited number and size of trials provided insufficient data to draw conclusive evidence on the effectiveness of homeopathy for most conditions.”
• Cochrane Review 2004.
Six randomized placebo controlled trials covering 556 people with stable chronic asthma were included. None of the trials reported significant differences on validated symptom scales. The authors concluded: “There is not enough evidence to reliably assess the possible role of homeopathy in asthma.”
• Lancet 2005.
One hundred and ten studies were analyzed. In two-thirds of these homeopathy was more effective than placebo, in less than ten percent it was less effective than placebo and in the remainder of the studies (25%) it was equally effective. The authors of this article chose eight studies from the second group to be included in their meta-analysis, ignoring the majority of studies with positive results. They unsurprisingly concluded that: “there was weak evidence for a specific effect of homoeopathic remedies…This finding is compatible with the notion that the clinical effects of homoeopathy are placebo effects.” Obviously, this report was biased in its failure to include all the data.
• Cochrane Review 2006.
The authors evaluated 7 studies of Oscillococcinum for the treatment (4 studies, n=1,194) and prevention (3 studies, n=2,265) of influenza. Treatment studies revealed a reduction of symptom duration by 0.28 days (95% CI). The authors concluded that: “Though promising, the data were not strong enough to make a general recommendation to use Oscillococcinum for first-line treatment of influenza and influenza-like syndromes. Further research is warranted but the required sample sizes are large.”
• Journal of the Faculty of Homeopathy 2006.
Authors reviewed 8 RCTs using homeopathy to treat anxiety and anxiety disorders. Several uncontrolled and observational studies reported positive results including high levels of patient satisfaction. Because of the lack of control groups, it was difficult to assess the extent to which any response was due to homeopathy. The authors concluded that: “On the basis of this review it is not possible to draw firm conclusions on the efficacy or effectiveness of homeopathy for anxiety. However, surveys suggest that homeopathy is quite frequently used by people suffering from anxiety. If shown to be effective, it is possible that homeopathy may have benefits in terms of adverse effects and acceptability to patients…. further investigation is indicated.”
• Mayo Clinic Proceedings 2007.
The authors reviewed 16 out of 326 available trials for inclusion in their analysis. The evidence for ADHD and Childhood Diarrhea show mixed results, while the remainder of the studies did not yield convincing evidence to support the use of homeopathy. The authors concluded: “The evidence from rigorous clinical trials of any type of therapeutic or preventive intervention testing homeopathy for childhood and adolescence ailments is not convincing enough for recommendations in any condition.”
This review indicates that although study flaws do exist, a significant portion of trials and meta-analyses of trials support the theory that homeopathy is more than just a placebo response.
H-The Safety of Homeopathy
Americans fill an estimated 3.7 billion prescriptions annually as well as purchasing a multitude of over the counter (OTC) preparations and herbal supplements. In 2004, 82% of the U.S. population reported taking at least one prescription drug, OTC preparation or dietary supplement on a regular basis. Thirty percent reported the use of 5 or more of these on a regular basis.
In the elderly population 75% of those over age 65 took 4 or more prescription medications per day. The average 75 year-old took 8 prescription medications daily.
Individuals over age 65 were more than twice as likely to be treated in the Emergency Room for adverse drug effects than younger subjects and nearly 7 times as likely to require hospitalization for these events.
Studies from the federal Centers for Disease Control and Prevention (CDCP), the Food and Drug Administration (FDA) and the US Consumer Product Safety Commission (USCPSC) indicate that an average of 10% of all hospital admissions in the U.S. are attributable to drug-induced disorders and side effects. Approximately 1 million hospitalized patients are injured from drugs each year and 180,000 die as a result of these injuries.
The AMA considers conventional allopathic medicines to be a “major” cause of serious illness, even when used according to current guidelines. These injuries are “dose dependent and predictable”, and many are the result of drug-drug, drug-disease and drug-food interactions.
The Institute of Medicine (IOM) reports that the cost of allopathic medication errors in the U.S. alone tops $3.5 Billion per year.
Adverse drug reactions (ADRs) appear to be between the 4th and 6th leading cause of death in the U.S. today. Data reveals that the majority of these deaths take place when patients are taking medications “correctly” as prescribed by their physicians.
When homeopathic medicines are prescribed according to Classical Homeopathic guidelines and the Law of Similars, they lack the potential for serious and life threatening side effects.
Investigations by the FDA uncovered very few reports of illness associated with the use of homeopathic remedies. Reports filed with poison control centers and regulatory agencies determined that homeopathic remedies are only “infrequently” involved in any adverse reactions. Many of these reports actually overestimate the role of homeopathic side effects because of the institutional confusion that exists in correctly differentiating between homeopathic and herbal medicines. Most of the public and even professionals working in these institutions are unaware of the differences between these categories of agents.
A review performed by the Royal London Homeopathic Hospital surveying all published works between 1970 and 1995, including a comprehensive worldwide literature search and inquiries into regulatory agencies and manufacturers of homeopathic products revealed that the adverse effects from homeopathic treatment were consistently “mild and transient”. The most common symptoms reported were headache, fatigue, rash, vertigo, and diarrhea. These adverse effects were reported at about the same frequency as side effects from placebo.
A recent prospective observational tracking study of more than one thousand acute prescriptions at the Glasgow Homeopathic Hospital recorded adverse events or associations at less than 2%.
Some patients receiving homeopathic treatment do report feeling worse for a brief period of time after starting homeopathic remedies. Homeopaths label this as an “initial aggravation” and interpret it positively as a sign that the remedy is in tune with the body, temporarily stimulating the aggravation of symptoms in the process leading to the restoration of health.
The healing reaction provoked by homeopathy can also lead to a temporary recurrence of old symptoms from previous diseases, which then self-resolve. Patients report occurrence of these side effects at about the same frequency as those who are given placebo.
Unscrupulous individuals or groups who produce and market products falsely labeled as “homeopathic” may also be responsible for some of the few adverse effects reported. Some so-called homeopaths may even recommend the misapplication or incorrect use of reputable homeopathic products. Strictly adhering to a Classical Homeopathic protocol for treatment provided by a licensed health care provider is one way of assuring the greatest possible benefits and the least possible side effects and complications from homeopathic treatment.
Homeopathic remedies do not appear to interfere with conventional allopathic drugs. They are not responsible for drug – drug interactions. They also do not interact with each other or with foods and beverages, as do allopathic medications. This is because Homeopathic medicines do not act by herbal, pharmaceutical or other known chemical mechanisms.
There is no doubt that the safety profile of homeopathic medicines is vastly superior to that of allopathic drugs.
I- Conclusion
Homeopathy is a comprehensive system of medicine based on a scientific study of drugs, drug reactions and individual cases of cure. This data establishes that homeopathy works. However, because the mechanism by which homeopathy works is not well understood and because provisional explanations do not match the scientific perspective of allopathic researchers, great controversy and animosity between homeopathic and allopathic practitioners has been generated.
What we do understand can be summarized as follows:
- Homeopathy (when utilized with integrity and respect for homeopathic guidelines) has clinical effects over and above those of placebo.
- Homeopathy is useful in a broad range of conditions.
- Patients are usually extremely satisfied with the results of homeopathic treatment.
- The safety profile of homeopathic medicines is excellent.
- Homeopathic treatment is cost effective.
- More research is required to substantiate its effectiveness and discover its mechanism of action.
- The adoption and integration of homeopathy into mainstream practice is justified.
Homeopathy is a centuries-old tradition of medicine that is poised to re-invigorate the failing practice of conventional allopathic medicine. There is no doubt that increased research funding is necessary for homeopathy to be able to do this. Efforts to integrate homeopathic training into the university curriculum should be strongly supported in this process. Even without such research the safety and efficacy of homeopathy are already well established. This means that its evidence-based benefits are now available for patient and physician alike.
“What then should be our attitude toward homeopathy? Rather than stressing its implausibility and the notion that its practice fits the definition of quackery or represents a cult, we might prefer to opt for a more constructive approach….Until such evidence is available, we ought to keep an open mind and remember that a treatment might work even if we fail to understand why.”
This paper was first presented at the Systems and Symbiosis Conference hosted by New York Medical College, The American Institute of Homeopathy and The Homeopathic Medical Society of the State of New York in Tarrytown, New York on October 23, 2008.
Ronald D. Whitmont, MD is Clinical Assistant Professor of Family and Community Medicine at New York Medical College, Valhalla, New York. He is president of the Homeopathic Medical Society of the State of New York (HMSSNY) and Treasurer of the American Institute of Homeopathy (AIH). He maintains a private practice of Classical Homeopathic Medicine in Rhinebeck and New York City, New York.
Appreciation to my brother Andrew D. Whitmont, PhD, clinical psychologist in Yakima, Washington, for his helpful editorial modifications.
References
- Parker-Pope T. Well. Doctor and Patient, Now at Odds, The NYTimes, July 29, 2008:F6.
- Rosenberg MT. Is There a Doctor in the House? President’s Column. Medical Society of the State of New York, News of New York. 2008; 63(8):4.
- http://www.aamc.org t/newsroom/reporter/july2000/woes.htm
- Am Fam Phys. AAFP New Now. 2009;79(1):5.
- www.physiciansfoundation.org
- Henry TA. State societies warn of primary care shortages. Am Med News. 2008. December :12.
- Sorrel AL. Defensive medicine widespread among Mass. Doctors. Am Med News. 2009, Jan5:9.
- Eisenberg DM, et al. Trends in Alternative Medicine Use in the United States, 1990-1997 Results of a Follow-up National Survey JAMA. 1998;280:1569-1575..
Cassedy JH. American Medicine and Statistical Thinking, 1800-1960. Cambridge, Mass.Harvard university Press. 1984. - http://www.oecd.org/health/healthdata
- Access to Care in Crisis: Physicians in Short Supply. HANYS’ 2008 Physician Workforce Study. Medical Society of the State of NY, News of NY, 2009;63(12):1&8.
- Wolf U, et al. Prevalence, use, effectiveness and appreciation of CAM among patients and physicians in Switzerland. A search of the current literature… 12th Annual Symposium on Complementary Health Care — Abstracts: 19th-21st September 2005, Exeter, UK. Focus on Alternative & Complementary Therapies (FOCUS ALTERN COMPLEMENT THER), 2005; 10: Supplement 1: 58-9.
- Spence DS, Thompson EA, Barron SJ. The Journal of Alternative and Complementary Medicine. October 1, 2005, 11(5): 793-798.
- The evidence base of complementary medicine 2nd Edition, The Royal London Homeopathic Hospital, 1999.
- Walach H,. Jonas WB, Ives J, Van Wijk R, Weingartner O. The Journal of Alternative and Complementary Medicine. October 1, 2005, 11(5): 813-829.
- Model Guidelines for the Use of Complementary and Alternative Therapies in Medical Practice, Approved by the House of Delegates of the Federation of State Medical Boards of the United States, Inc. Special Committee for the Study of Unconventional Health Care Practices (Complementary and Alternative Medicine): 2001-2002.
- Sackett DL, Rosenberg WM, Gray JA, et al. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71-72.
- Benefits and risks of homoeopathywww.thelancet.com; 370 November 17, 2007: 1672-73.
- BMJ Clinical Evidence handbook, BMJ Publishing Group, London, UK, 2007.
- http://clinicalevidence.bmj.com/ceweb/about/knowledge.jsp
- [1] Ezzo J, Bausell B, Moerman DE, Berman B, Hadhazy V. Reviewing the reviews. How strong is the evidence? How clear are the conclusions? Int J Technol Assess Health Care. 2001 Fall;17(4):457-66.
- http://nytimes.com. April 22, 1986.
- http://www.wired.com/medtech/health/news/2005/07/68153.
- Martin B. Suppressing Research Data: Methods, Context, Accountability, and Responses. Accountability in Research, Vol. 6, 1999, pp. 333-372.
- Saul S. Experts Conclude Pfizer Manipulated Studies. An Epilepsy Drug Known for Off-Label Uses. NYT Business, 2008;October 8:B4.
- Moncrief J, Kirsch I. Efficacy of antidepressants in adults. BMJ 2005;331:155-7.
- Harris G. F.D.A. Is Lax On Oversight During Trials, Inquiry Finds. NY Times. 2009, Jan 12: A10.
- Landers SJ. Data from drug trials often not published. Am Med News, 2008, Oct 13: 24-25.
- Wilson D. Investigation Links Wyeth to Articles on Its Drug. NY Times. 2008, Dec 13: B1, B8.
- DeAngelis CD; Fontanarosa PB. Impugning the integrity of medical science: the adverse effects of industry influence.Preview JAMA: Journal of the American Medical Association, 2008 Apr 16; 299.
- Landers SJ. Studies suggest drugmaker skewed clinical trial reports. Am Med News. 2008, May 12:21-22.
- Harris G. Drugmakers Paid Radio Host $1.3 Million for Lectures. NY Times. 2008, Nov 22:A14.
- Harris G. In Documents, Ties Between Child Psychiatry Center and Drug Maker. NY times 2008, Nov 25: A22.
- http://www.fhsc.salford.ac.uk/hcprdu/projects/homeopathic.htm.
- Mathie RT. The research evidence base for homeopathy: a fresh assessment of the literature. Homeopathy 2003; 92: 84-91.
- Culliton BJ. The academic-industrial complex. Science. 216(4549):960-2, 1982 May 28.
- Pharmaceutical R&D Spending: Pharmaceutical Research and Manufacturers of America, PhRMA Annual Survey, 2001. NIH Budget. www.nih.gov, 2001.
- Abramson J, Starfeld B. The Effect of Conflict of Interest on Biomedical Research and Clinical Practice Guidelines: Can We Trust the Evidence in Evidence-Based Medicine? JABFP September–October 2005;18(5):414-18.
- Vandenbroucke JP, Commentary. Homeopathy trials: going nowhere. Lancet. 1997;350:824.
- Walsh N, Alternative Medicine, An Evidence-Based Approach. Homeopathy: Does it Have a Role?. Int Med News; Nov 1, 2001:13.
- Langman MJS, Commentary. Homeopathy trials: reason for good ones but are thy warranted? 1997;350:825.
- Coulter, HL, Divided Legacy, Vol 1, North Atlantic Books, Berkeley, CA.
- http://www.chedu.org.
- http://www.amcofh.org/
- www.thelancet.com; 370 November 17, 2007:1677-78.
- Cassedy JH. American Medicine and Statistical Thinking, 1800-1960. Cambridge, Mass.Harvard university Press. 1984.
- Weatherley-Jones E., Thompson EA, Thomas KJ. The placebo-controlled trial as a test of complementary and alternative medicine: observations from research experience of individualized homeopathic treatment. Homeopathy 2004; 93: 186-9.
- http://www.lmhint.net
- Bell IR The Evolution of Homeopathic Theory-Driven Research and the Methodological Toolbox. American Homeopath. 2008:56-74.
- Milgrom LR, Is homeopathy possible? J R Soc Health. 2006;126(5):211-8,
- Linde K, Jonas WB, Melchart D, and Willich S. The methodological quality of randomized controlled trials of homeopathy, herbal medicines and acupuncture Int. J. Epidemiol., Jun 2001; 30: 526 – 531.
- Reilly D, Taylor MA, Beattie NGM, et al. Is evidence for homeopathy reproducible? Lancet 1994;344:1601-6.
- Matusiewicz R. The effect of a homeopathic preparation on the clinical condition of patients with corticosteroid-dependent bronchial asthma. Biomed Ther 1997;15:70-4.
- Keil T. Witt CM. Roll S. Vance W. Weber K. Wegscheider K. Willich SN. Homoeopathic versus conventional treatment of children with eczema: a comparative cohort study. Complementary Therapies in Medicine. 2008 ;16(1):15-21.
- Jacobs J, Jiminez LM, Gloyd SS, et al. Treatment of acute childhood diarrhea with homeopathic medicine: a randomized clinical trial in Nicaragua. Pediatrics 1994;93:719-25.
- Jacobs J, Jimenez LM, Malthouse S, et al. Homeopathic treatment of acute childhood diarrhea: results from a clinical trial in Nepal. J Altern Complement Med 2000;6:131-9.
- Friese KH, Kruse S, Ludtke R, Moller H. The homeopathic treatment of otitis media in children- comparisons with conventional therapy. Int J Clin Pharmacol Ther 1997;35:296-301.
- Jacobs J, Springer DA, Crothers D. Homeopathic treatment of acute otitis media in children; a preliminary randomized placebo –controlled trial. Pediatr Infect Dis J 2001;20:177-83.
- Frei H. Thurneysen A. Homeopathy in acute otitis media in children: treatment effect or spontaneous resolution? British Homoeopathic Journal. 2001; 90(4):180-2.
- Reilly DT, Taylor MA. Potent placebo or potency? A proposed study model with initial findings using homoeopathically prepared pollens in hayfever. Br Homeopath J 1985;74(2):65-75.
- Taylor MA, Reilly D, Llewellyn-Jones RH, et al. Randomised controlled trial of homoeopathy versus placebo in perennial allergic rhinitis with overview of four trial series. Br Med J 2000;321(7259):471-76.
- Ferley JP, Zmirou D, Adhemar D, Balducci F. A controlled evaluation of a homeopathic preparation in the treatment of influenza-like syndromes. Br J Clin Pharmacol 1989;27:329-35.
- Papp R, Schuback G, Beck E, et al. Oscillococcinum in patients with influenza-like syndromes; a placebo-controlled double-blind evaluation. Br Homeopath J 1998;878:69-76.
- Tveiten D. Bruset S. Effect of Arnica D30 in marathon runners. Pooled results from two double-blind placebo controlled studies. Homeopathy: the Journal of the Faculty of Homeopathy.2003;92(4):187-9.
- Stam C, Bonnet MS, van Haselen RA. The efficacy and safety of a homeopathic gel in the treatment of acute low back pain: a multi-centre, randomized, double-blind comparative clinical trial. Br Homeopath J 2001;90:21-8.
- Gmunder R. Kissling R. [The Efficacy of homeopathy in the treatment of chronic low back pain compared to standardized physiotherapy] Zeitschrift fur Orthopadie und Ihre Grenzgebiete. 2002;140(5):503-8.
- Balzarini A, Felisi E, Martini A, De Conno F. Efficacy of homeopathic treatment of skin reactions during radiotherapy for breast cancer: a randomized, double-blind clinical trial. Br Homeopath J 2000;89:8-12.
- Kulkarni A, Nagarkar BM, Burde GS. Radiation protection by use of homeopathic medicines. Hahnemann Homoeopath Sand 1998;12:20-3.
- Gibson ERG, Gibson SL, MacNeill AD, Buchanan WW. Homeopathic therapy in rheumatoid arthritis: evaluation by double-blind clinical therapeutic trial. Br J Clin Pharmacol 1980;9:453-9.
- Chapman EH, Weintraub RJ, Milburn MA, eta al. Homeopathic treatment of mild traumatic brain injury: A randomized, double –blind, placebo-controlled clinical trial. J Head Trauma Rehabil 1999;14:521-42.
- De Lange de Klerk ES, Blommers J, Kuik DJ, eta al. Effect of homeopathic medicines on daily burden of symptoms in children with recurrent upper respiratory tract infections. B r Med J 1994;309:1329-32.
- Diefenback M, Schilken J, Steiner G, Becker HJ. Homeopathic therapy in respiratory tract diseases. Evaluation of a Clinical study in 258 patients. Zeitschr Fur Allgemeinmedizin 1997;73:308-14.
- Riley D. Fischer M. Singh B. Haidvogl M. Heger M. Homeopathy and conventional medicine: an outcomes study comparing effectiveness in a primary care setting. J Altern Complement Med 2001;7(2):149-59.
- Aickin M. The end of biomedical journals: there is madness in their methods. Journal of Alternative & Complementary Medicine 2005; 11 (5):755-7.
- Kleijnen J, Knipschild P, Ter Riet G, Clinical trials of homeopathy. Br Med J 1991;302(6772):316-23.
- Boissel JP, Cucherat M, Haugh M, Gauthier E. Critical literature review on the effectiveness of homoeopathy: overview of data from homoeopathic medicine trials. Brussels, Belgium: Homoeopathic Medicine Research Group. Report to the European Commission. 1996: 195–210. http://www.unconventional-medicine.com/wwuna.htm
- Linde K, Clausius N, Ramirez G, et al, Are the clinical effects of homeopathy placebo effects? A meta-analysis of placebo–controlled trials.Lancet 1997;350:834-43.
- Linde K, Melchart D. Randomized controlled trials of individualized homeopathy: a state-of-the-art review. J Alter Complement Med 1998; 4: 371–88.
- Cucherat, M., Haugh, M.C., Gooch, M., and Boissel, J.-P. “Evidence of Clinical Efficacy of Homeopathy: A Meta-Analysis of Clinical Trials.” European Journal of Clinical Pharmacology. 2000. 56(1):27-33.
- Jonas WB, Kaptchuk TJ, Linde K. A Critical Overview of Homeoopathy. Ann I ntern Med. 2003:138:393-399.
- Cochrane Database Syst Rev. (1):CD000353, 2004.
- Shang A, Huwiler-Muntener K, Nartey L, Juni P, Dorig S, Sterne JA, Pewsner D, Egger M. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. Lancet. 2005; 366 (9487):726-32.
- Homoeopathic Oscillococcinum for preventing and treating influenza and influenza-like syndromes.[update of Cochrane Database Syst Rev. 2004;(1):CD001957.
- Pilkington K. Kirkwood G. Rampes H. Fisher P. Richardson J.Homeopathy: the Journal of the Faculty of Homeopathy. 2006 95(3):151-62.
- Altunc U. Pittler MH. Ernst E., Homeopathy for childhood and adolescence ailments: systematic review of randomized clinical trials. Mayo Clin Proc 2007;82(1):69-75.
- Manasse HR. Medication use in an imperfect world: drug misadventuring as an issue of public policy, Part 1. Am J Hosp Pharm 1989;46:929-44.
- Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, et al. Incidence of adverse drug events and potential adverse drug events . Implications for prevention. JAMA 1995;274:29-34.
- Holland EG, Degruy FV. Drug-Induced Disorders. Am Fam Phys. 1997:1781-88.
- Lazarou J, Pomeranz BH, Corey PN. Incidence of Adverse Drug Reactions in Hospitalized Patients. A Meta-analysis of Prospective Studies. JAMA 1998;279(15):1200-5.
- Markel H, Marsh B. How Two Rights Can Make a Wrong. NYT 2007, Feb 25:5.
- http://nccam.nih.gov/health/homeopathy
- Dantes F, Rampes H, Do homeopathic medicines provoke adverse effects? Conference proceedings: Improving the success of homeopathy. London Royal London Homeoapthic Hospital. 1999p70-74. and Br Homeopathic J. 2000;89(Supl):535-538.
- Reilly D Homeopathy: Increasing Scientific Validation. Altern Ther Health Med. 2005;11(2):28-31.
- Song EH. Homeopathy as a Treatment Option. Southern California Physician. 2008; Dec:6-7. www.socalphys.com
- Ernst E. Commentary: Homeopathy Revisited. Arch Intern Med 156. 1996:2162-64.
- http://www.homeopathyusa.org
- http://www.hmssny.org
- http://www.homeopathicmd.com

